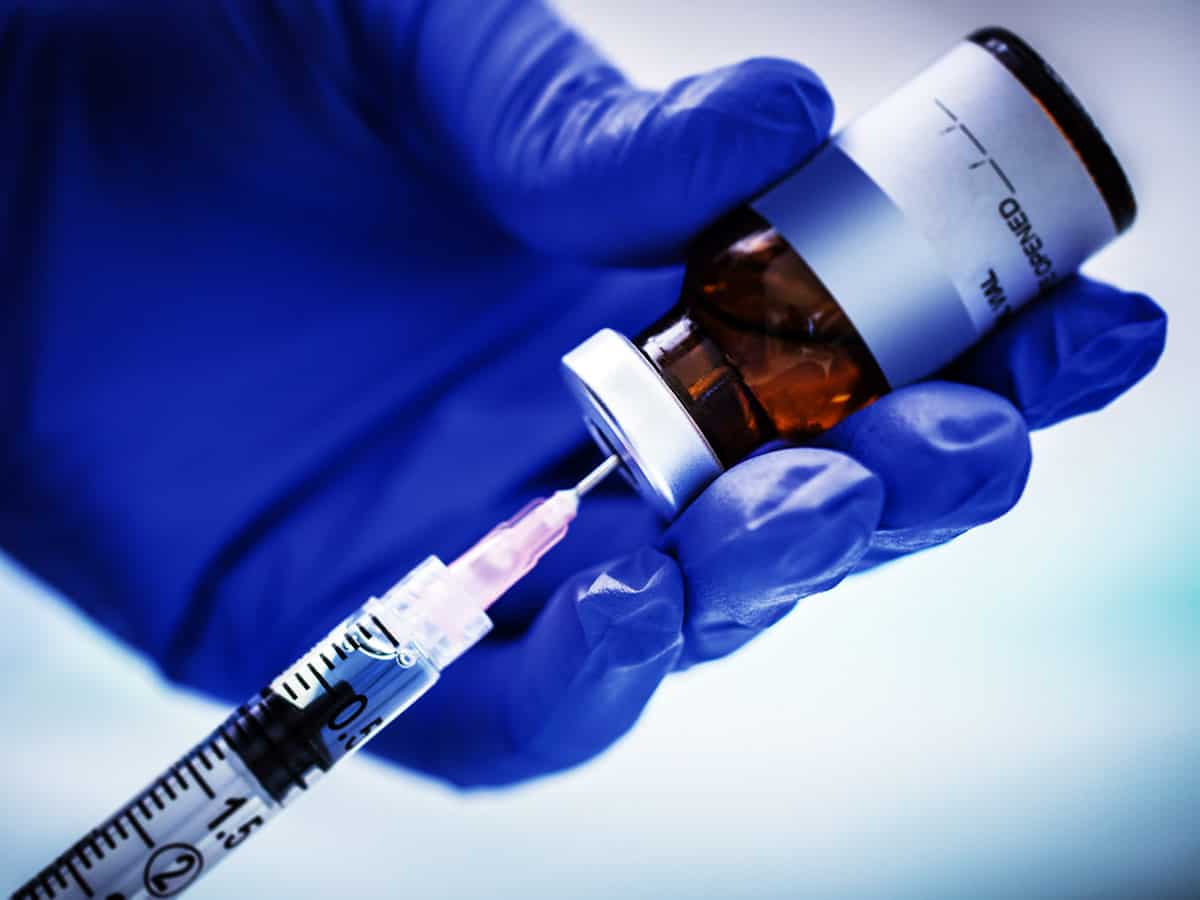
Biotechnology company Moderna developed the COVID-19 vaccine in partnership with the National Institutes of Health, and the phase 1 study of it has been found to induce immune responses in all of the volunteers. The vaccine is ready to go for final testing, a large Phase 3 trial by July end.
The vaccine is said to be working on inducing immune response with mild side effects like chills, headache, muscle pain, fatigue, and pain at the injection spot. The company said it will be able to deliver around 500 million doses per year and may go up to 1 billion doses per year from 2021.
According to the Phase 1 study, the company’s goal was safety and then the immune responses. Well, one senior investigator from Seattle who was involved in the study opined that the immune responses are promising but it is not known yet if the levels seen in the study would actually protect against infection. It can only be known from an actual efficiency trail.
Phase 1 study usually studies on a small group and to determine if the developed vaccine is safe and if it is inducing the immune response. Phase 2 is the clinical study on the vaccine given to the people with age and physical health characteristics similar to those the vaccine is actually intended, as per CDC. Phase 3 is on the larger group, typically thousands to test the efficacy and safety as well.
Tags COVID-19 Vaccine Moderna Coronavirus Vaccine US Coronavirus Vaccine
 Gulte Movie News And Politics
Gulte Movie News And Politics