
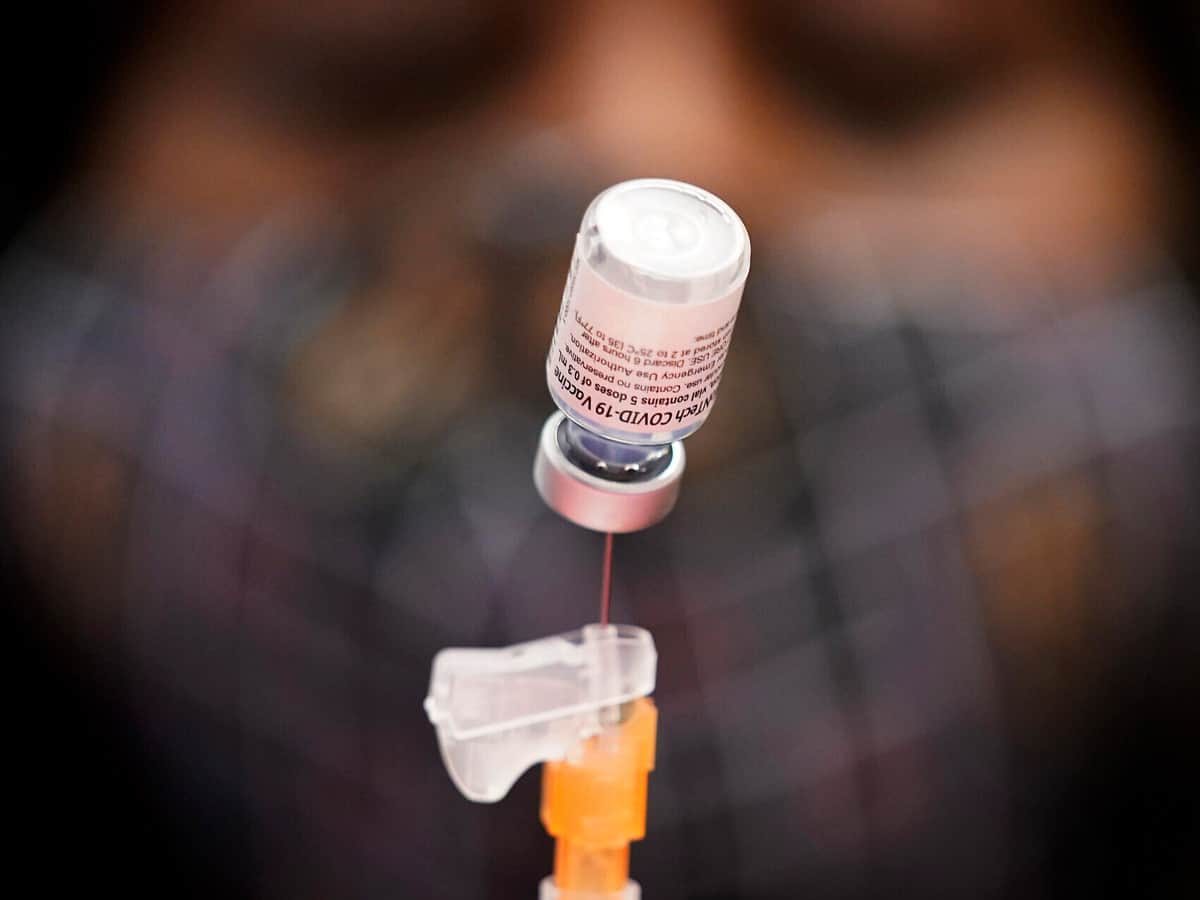
Historically, most therapeutics do not include the pediatric population in the initial trials. However, Pfizer announced on Wednesday that its COVID-19 vaccine is safer and effective even in children as young as 12. Pfizer said hopefully they can begin giving shots to young kids before they head back to school next academic year.
As we know, many vaccines are being rolled out global-wide for adults so far. Now, this is a challenging process involving children and the Pfizers vaccine is authorized for ages 16 and older. With successful children vaccines, it can also eradicate the pandemic and help with their normal academic years ahead. Or, at least the high school students will start to look a little more normal after months of disruption.
While trails and giving shots for this particular age groups should not be rushed, it is also cardinal to see how well the shots revved up the kids immune systems. Researchers said that children will have similar side effects as adults like body pains, fever and fatigue. However, kids have more virus-fighting antibiotics. It is reported that both Pfizer and its German partner BioNTech would soon take a step in asking the US Food and Drug Administration and European regulators to allow emergency use of the shots starting at age 12.
Pfizer CEO Albert Bourla said that there is a need to expand the vaccine as soon as possible. Last month, AstraZeneca started a study of its vaccine among 6 to 17 years age group in Britain. Parallel to that, Johnson & Johnson is planning on its own pediatric trials. Even China’s Sinovac announced its submission on preliminary data to Chinese regulators showing its vaccine is safe in children as young as 3.
This post was last modified on 31 March 2021 7:29 pm
KGF fame Yash is busy with his upcoming film Toxic. Directed by Geethu Mohandas, the…
In yet another case of misleading information and misinterpretation, popular Telugu character artiste Gayatri Bhargavi…