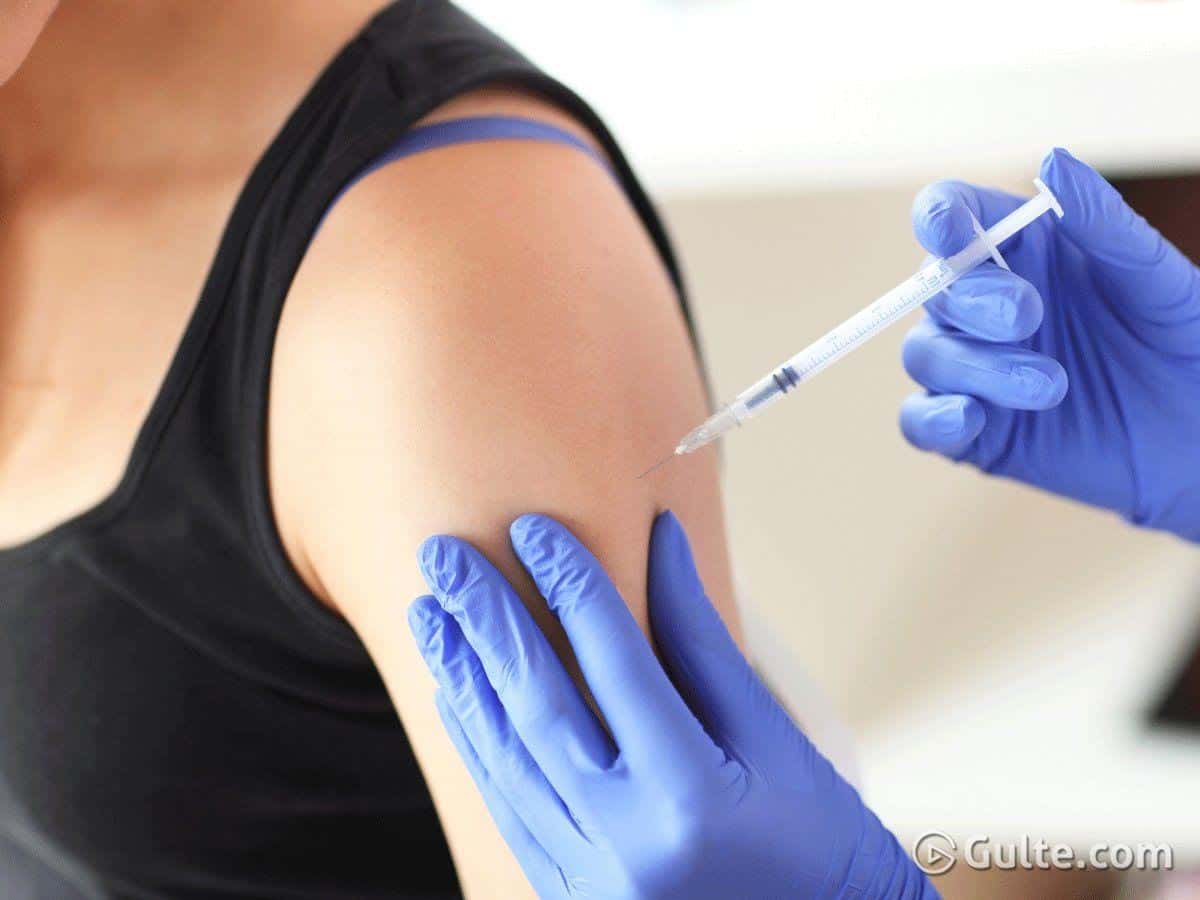
The Phase 3 clinical trials of Oxford vaccine, AZD 1222 Covid-19, have been temporarily stalled. Oxford vaccine is the frontrunner in the race for the Covid vaccine. There is information that a trial participant from Britain who took a shot of the vaccine developed severe complications following which AstraZeneca declared that it had temporarily halted the human trials of the vaccine which had reached the final stage. The pharma company said that they had stalled the trials to review the safety data. However, it did not reveal the health complications the volunteer developed.
The British-Swedish pharma company said that such hurdles were common in the development of vaccines. When such situations arise, there should be an in-depth review of the whole process and then the experiments are taken forward, a representative of AstraZeneca said.
Integrity of trials
This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials to maintain integrity of the trials. When trials are being taken up on a large-scale, there is possibility of illnesses among 1-2 volunteers. The problem will be independently checked and then the trials will be continued very soon, the pharma firm said.
Responding to the development, several scientists across the world said the health complication could not have been a regular fever or cold but something more intense forcing the trial participant to get admitted in a hospital. It’s crucial to show whether the person’s reaction was caused by the vaccine, or just a coincidence.
However, they said that there was nothing much to worry over the pause button pressed on the trials. The company spokeswoman Michele Meizell said: “Our standard review process was triggered and we voluntarily paused vaccination to allow review of safety data by an independent committee.”
Another scientist from Columbia University felt that the health complication in the trial participant could not have been due to the vaccine. Whatever could be the reason, clinical trials are held to identify such issues. In the last stage, the vaccine shots are administered to a large number of volunteers to see if any adverse impact is there which could be identified in the initial phases.
Major hiccup
AstraZeneca had taken 30,000 volunteers onboard for the phase 3 clinical trials in America. It is learnt that of them, around 300 were given the vaccine shots. When the vaccine is experimented on such a large number of people, the results will be clear.
This interruption is the first major hiccup in what has been a remarkably smooth path in the rapid vaccine effort going across the globe. It is usually in the large-scale, make-or-break Phase 3 clinical trials that real issues are most likely to occur.
Oxford-AstraZeneca’s AZD 1222 is one among the nine potential vaccines currently undergoing phase 3 clinical trials around the world. Among them, the vaccines being developed by Moderna and Pfizer are in an advanced stage.
It is significant to note that this interruption in the trials occurred hours after several institutions involved in the development of the vaccine had adopted a resolution not to compromise on the standards in the development of the vaccine and its safety.
Meanwhile India has touched the 43 Lakh mark of the total positive cases. Freshly 89,706 cases have been reported while the total cases count is at 43,70,219. 1115 people have died in the last 24 hours and the total death tally is 73,890.
11,54,549 samples were tested yesterday and a total of 5,18,04,677 samples tested up to 8th September, said the ICMR.
Tags Corona Oxford Vaccine Covid-19
 Gulte Movie News And Politics
Gulte Movie News And Politics